Question 1.
Give an example of a metal which
(a) is a
liquid at room temperature
(b) can be
easily cut with a knife !
(c) is the
best conductor or heat
(d) is the
poorest conductor of heat.
Answer.
(a) Mercury
(b) Sodium
(c) Silver/copper
(d) Lead/mercury
Question 2.
Explain the meaning of malleable and ductile.
Answer.
Malleable The
property due to which a substance can be beaten into thin sheet is known as
malleability. For example, gold and silver
Ductile The
property due to which a substance can be drawn into thin wires is known as
ductility. Many metals are ductile in nature. For example, gold and silver
Question 3.
Why is sodium kept immersed in kerosene
oil?
[2011]
Answer.
It is a highly reactive metal. Sodium
reacts both with air and water. When kept in open, it readily combines with
the oxygen present in air to form its oxide. Similarly, it reacts with water
or moisture to form sodium hydroxide.
4Na(s) + O
2(g) ➝ 2Na
2O(s)
2Na(s) + 2H
2O(l) ➝ 2NaOH(aq) + H
2(g)
In order to preserve sodium metal, we
generally keep it under kerosene so that neither air nor moisture comes in
contact with it.
Question 4.
Write the equations for the reactions of:
(a) iron with
steam
(b) calcium
until water
Answer.
(a) 3Fe(s) +
4H
2O(g) ➝ Fe
3O
4(s) + 4H
2(g)
(b) Ca(s) +
2H
2O(7) ➝ Ca(OH)
2(aq) + H
2(g)
Question 5.
Samples of four metals A, B, C and D were
taken and were added to the following solutions one by one. The results
obtained have been tabulated and answer the questions that follows:
| Metal | Solution in which metal is added | |||
|
Iron(II) |
Copper(II)
|
Zinc
|
Silver
nitrate |
|
| A | No reaction | Displacement | – | – |
| B | – | No reaction | – | – |
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
Answer.
Based on the activity series, the relative
position of the metals involved in solutions is: Zn > Fe > Cu > Ag.
On the basis of the results given in the table.
- Metal ‘A’ is more reactive than copper and less reactive than iron.
- Metal ‘B’ is more reactive than iron and less reactive than zinc.
- Metal ‘C’ is more reactive than silver and less reactive than copper.
- Metal ‘D’ is equally or less reactive than silver. In the light of above the information, we can conclude that:
(a) Metal ‘B’
is the most reactive.
(b) Since ‘B’
is more reactive than iron, it is also more reactive than copper. This means
that it would displace copper from copper(II) sulphate solution. The blue
colour of solution will slowly fade.
(c) The
decreasing order of reactivity of metals is: B > A > C > D.
Question 6.
Which gas is produced when dil.
hydrochloric acid is added to a reactive metal? Write the chemical reaction
when Iron reacts with dil. H
2SO4.
[2010]
Answer.
Hydrogen gas (H
2) is produced when a reactive metal reacts with dil. hydrochloric acid. Iron
and dil. H
2SO4 react as follow:
Fe(s) + H
2SO
4(dil.) ➝ FeSO
4(aq) + H
2(g)
Question 7.
What would you observe when zinc is added
to a solution of iron(II) sulphate? Write the chemical reaction that takes
place, [2010] Ans. The green colour of the solution would slowly disappear.
Zinc would gradually dissolve and iron would get precipitated at the bottom of
the beaker. The reaction that takes place is:
Zn(s) + FeSO
4(aq) ➝ ZnSO
4(aq) + Fe(s)
Question 8.
(a) Give
electron dot structures for sodium, magnesium and oxygen.
(b) Show the
formation of Na
2O and MgO by the transfer of electrons.
(c) What are
the ions present in these compounds?
Answer.

Question 9.
Why do ionic compounds have high melting –
points?
Answer.
In the formation of ionic compounds,
positive ions (cations) and negative ions (anions) participate. These are
closely packed and the ionic compounds exist as crystalline solids.
They have strong inter ionic forces of
attraction and have high melting and boiling points.
Question 10.
Define the following terms:
(a) Minerals
(b) Ores
(c) Gangue
Answer.
(a) Minerals
The naturally occurring com-pounds of metals along with some impurities are
called minerals.
(b) Ores The
minerals from which metals can be conveniently and profitably extracted are
called ores.
(c) Gangue
The associated impurities of minerals and ores are called gangue.
Question 11.
Name two metals which are formed in nature
in free state.
Answer.
The metals are gold (Au) and platinum (Pt).
Question 12.
Which chemical process is used for
obtaining a metal from its oxide?
Answer.
The chemical process is known as reduction.
Question 13.
Metallic oxides of zinc, magnesium and
copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide | |||
| Magnesium oxide | |||
| Copper oxide |
In which cases, will you find displacement reactions taking place?
Answer.
Magnesium (Mg) will displace both zinc (Zn)
and copper (Cu) from their oxides
Mg + ZnO
➝ MgO + Zn
Mg + CuO ➝ MgO + Cu
Zinc will displace copper from copper
oxide.
Zn + CuO ➝ ZnO + Cu
Copper is the least reactive metal and will
not participate in the displacement reaction.
Question 14.
Which metals do not corrode easily?
Answer.
Metals such as gold (Au) and platinum (Pt)
present at the bottom of the reactivity series do not corrode easily.
Question 15.
What are alloys?
[2013]
Answer.
Alloys are the homogeneous mixture of two
or more metals, or even metals and non-metals.
Chapter End Questions
Question 1.
Which of the following will give
displacement reactions?
(a) NaCl
solution and copper metal
(b) MgCl
2 solution and aluminium metal
(c) FeSO
4 solution and silver metal
(d) AgNO
3 solution and copper metal
Answer.
(d) AgNO
3 solution and copper metal
Question 2.
Which of the following methods is suitable
for preventing an iron frying pan from rusting?
(a) applying
grease
(b) applying
paint
(c) applying
a coating of zinc
(d) all the
above.
Answer.
(c) applying
a coating of zinc
Question 3.
An element reacts with oxygen to give a
compound with high melting point. This compound is also water soluble. The
element is likely to be:
(a) Calcium
(b) Carbon
(c) Silicon
(d) Iron
Answer.
(a) Calcium
Question 4.
Food cans are coated with tin and not with
zinc because
(a) Zinc is
costlier than tin
(b) Zinc has
higher melting point than tin
(c) Zinc is
more reactive than tin
(d) Zinc is
less reactive than tin.
Answer.
(c) Zinc is
more reactive than tin
Question 5.
You are given a hammer, a battery, a bulb,
wires and a switch:
(a) How could
you use them to distinguish between samples of metals and non-metals?
(b) Assess
the usefulness of these tests to distinguish between ,metals and non-metals.
Answer.
(a) With the
help of a hammer, convert both the metal arid non-metal (solid) into plates or
rods. Metal will readily form these since they are malleable. Non-metals being
brittle will break, when struck with hammer. Now construct a cell in both the
cases using these plates as electrodes and switch on the current. If the bulb
glows, this means that the electrodes are of metals. In case it does not glow,
it means that the electrodes are of non-metals.
(b) From
these tests, we conclude that
- Metals are malleable while non-metals are not.
- Metals are good conductors of electricity while non-metals are not (graphite is an exception).
Question 6.
What are amphoteric oxides? Give examples
of two amphoteric oxides.
Answer.
These are oxides that can act both as acid
and base. For example, aluminium oxide (Al
2O
3) and zinc oxide (ZnO). The amphoteric character of zinc oxide is shown by
the following reactions.

Question 7.
Name two metals which can displace hydrogen
from dilute acids and two metals which cannot do so.
Answer.
Sodium and calcium can displace hydrogen
from dilute acids.
Copper and silver cannot
displace hydrogen from dilute acids.
Question 8.
In the electrolytic refining of metal M,
name anode, cathode and electrolyte.
Answer.
Anode: Rod of
impure metal
Cathode: Rod of
pure metal
Electrolyte:
Aqueous solution of soluble salt of metal M.
Question 9.
Pratyush took sulphur powder on a spatula
and heated it. He collected the gas evolved by inverting a test tube over it
as shown in the figure.
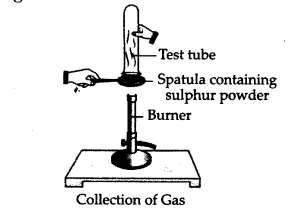
What will be the action of gas on
(a) dry
litmus paper?
(b) moist
litmus paper?
Write a balanced chemical
equation for the reaction taking place.
Answer.
(a) The gas
is sulphur dioxide (SO
2). It will not react with dry litmus paper.
(b) The gas
will bleach moist litmus paper. The moist litmus paper changes into red, as
the gas is dissolved in moisture to give sulphurous acid.
The balanced chemical equation involving
the formation of gas is:
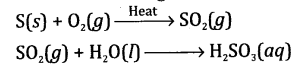
Question 10.
State two ways to prevent rusting of iron.
Answer.
Prevention of Rusting: It can be prevented by coating the metal surface with
- red lead
- paints
- enamel
- oil or grease
- plastic coating
- galvanising
- tinning
- electroplating with nickel or chromium
- converting iron into stainless steel.
Question 11.
What types of oxides are formed when
non-metals combine with oxygen?
Answer.
The oxides are generally acidic in nature
which means that when they are dissolved in water, their solutions change blue
litmus into red. For example,
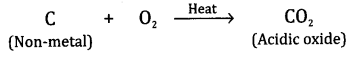
Question 12.
Give reasons for the following:
(a) Platinum,
gold and silver are used to make jewellery.
(b) Sodium,
potassium and lithium are stored under oil.
(c) Aluminium
is a highly reactive metal but still used for making cooking utensils.
(d) Carbonate
and sulphide ores are usually converted into oxides during the process of
extraction.
[2013,2014]
Answer.
(a) These
metals are placed at the bottom of the activity series and are least reactive
in nature. Gold and platinum are known as noble metals. They are not affected
by air, water or even by chemicals. Since they have lustre, jewellery can be
made from them.
(b) All three
metals react with water producing lots of heat. As a result, the hydrogen
evolved catches fire. They cannot be kept in air because air contains moisture
or water vapours. These are kept under kerosene to avoid contact with both air
and water.
(c) When
exposed to air, the metal changes into its oxide called aluminium oxide (Al
2O
3). It gets deposited over the surface of the metal and forms a protective
coating on the surface. Due to the presence of this layer, aluminium becomes
unreactive and can be used for making cooking utensils.
(d) Metal
oxides can be easily reduced to metallic form with coke (C) or any other
suitable reducing agent. Therefore, carbonates and sulphides are converted to
their oxide form by processes of calcination and roasting.
Question 13.
You must have seen tarnished copper vessels
being cleaned with lemon or tamarind juice. Explain why these sour substances
are effective in cleaning the vessels. [2014]
Answer.
Copper metal slowly reacts with water,
carbon dioxide and oxygen present in air to form a layer of basic copper
carbonate which is greenish in colour. This layer slowly gets deposited on the
surface of the metal.

Now lemon juice contains citric acid while tartaric acid is present in tamarind. Both these acids react with basic copper carbonate to form soluble salts such as copper acetate (with citric acid) and copper tartarate (with tartaric acid). The equations for the reactions are complicated and are not given. These salts gets removed from the surface of the copper metal and the surface of the metal shines.
Question 14.
A man went door to door posing as a
goldsmith. He promised to bring back the glitter on dull gold ornaments. An
unsuspecting lady gave a set of gold bangles to him which he dipped in a
particular solution. The bangles sparkled like new but their weight was
reduced drastically. The lady was upset but after a futile argument the man
beat a hasty retreat. Can you play the detective to find out the nature
of the solution he had used?
Answer.
The man had actually used the solution of
aqua regia (mixture of cone. HCl and cone. HNO
3 in the ratio of 3 :1 by volume) which has dissolved gold forming
soluble auric chloride (AuCl
3). Since gold actually reacted, there was a loss in weight of the gold
bangles. With the removal of the dull layer of gold from the surface, the
original shine on the bangles got restored.
Question 15.
Give reason why copper is used to make hot
water tanks and not steel (an alloy of iron).
Answer.
Copper is a better conductor of heat than
steel which is an alloy of iron. Due to this property, copper is used to make
water tanks for storing hot water, even though it is costlier than steel.
Question 16.
Differentiate between metals and non-metals
on the basis of chemical properties.
| S. No. | Property | Metals | Non-metals |
| 1. |
Electrochemical
|
Metals are electropositive in nature, i.e. they lose electrons to form
positively charged ions. For example,
|
Non-metals are electronegative in nature i.e. they accept electrons to
form negatively charged ions. For example,
|
| 2. | Nature of oxide |
They form basic oxides and if soluble in water give alkaline
solution.For example,
|
They form acidic or neutral oxides. When combined with water, they
from acids. For example, N 2O 5+H 2O⇌2HNO 3
|
| 3. |
Discharge of ions
|
On electrolysis, cations are discharged at the cathode. |
On electrolysis, anions are discharged at the anode.
|
| 4. |
Reaction with water/steam
|
Reactive metals like K, Na, Ca Al,
|
They, generally, do not react with water.
|
| 5. |
Reaction with dilute adds
|
They can displace hydrogen from acids and so form salts.
Zn + 2HCl(dil.) ➝ ZnCl 2+ H 2 Exception: Nitric acid (HN0 3) |
They do not react with dilute acids and hence do not displace hydrogen from acid.
|
| 6. |
Oxidising/ reducing behaviours
|
They act as reducing agents, i.e. they donate electrons.
|
They act as oxidising agents, i.e. they accept electrons.
|